Measurement of the Transfection Efficiency of Cultivated Cells
什么是转染?
Transfection is the process of introducing nucleic acids into animal cells to make the cells introduce specific genes to express a protein of interest. Nucleic acids introduced by transfection are referred to as foreign nucleic acids. Genes can be introduced to target cells without a virus, unlike with transduction, which requires a virus to transfer DNA.
核酸可以临时引入以表达蛋白质,或与宿主细胞的基因组一起复制。临时引入核酸被称为瞬时转染,同时用核酸复制转染称为稳定转染。因此,转染可用于增强或抑制转染细胞中的特定基因表达或产生重组蛋白。
转染原则
由结合蛋白质的脂质双层组成的细胞膜具有负电荷,可防止大型,带负电荷的核酸(例如DNA和RNA)通过。为了使转染成为可能,核酸必须以其他方式通过细胞膜。允许核酸通过细胞膜的其他方法包括化学,生物学和物理方法,每个方法都有不同的原理。本节使用将基因引入细胞为示例来解释每种方法。
化学转染
With chemical transfection, the negative charge of the nucleic acid is made positive or neutralized. Methods include lipofection and calcium phosphate co-precipitation, with lipofection being the most common.
LiPofection方法涉及通过带正电的合成脂质试剂头组和带负电荷的核酸之间的静电相互作用来构建阳离子脂质试剂复合物。阳离子脂质试剂复合物与负电荷的细胞膜表面相互作用,有效地将核酸引入细胞中。这使得细胞可以通过内吞作用在细胞质中吸收核酸。LiPofection是一种简单简单的转染方法,具有低毒性和出色的可操作性。
This method enables efficient uptake of both nucleic acids, DNA, and RNA. The lipofection method can also be used for both transient and stable transfections.
For chemical transfection, however, the conditions must be appropriate for the cell type and culture, resulting in a lower transfection efficiency than physical and biological methods.
生物转染
生物转染方法涉及病毒。基于病毒的转染具有高效,因为它利用了病毒的感染性。该方法用于对核酸摄取的细胞中蛋白质的过表达。该方法是体内转染的理想选择,使其成为临床研究最常用的方法。腺病毒,逆转录病毒和慢病毒用于体内基因摄取。
通过生物转染,在基因复制过程中会产生重组病毒,并在仅提取病毒载体之前用具有辅助功能的基因转染的细胞被接受并传播。然后生成包含要掺入基因的病毒载体,以将所需的遗传物质传递到细胞。
尽管基于病毒的转染具有高度适应活性生物的适应性,例如用于动物实验,但准备病毒可能很困难,并且需要遵守生物安全标准。免疫系统的插入突变和失活也有问题。
Physical transfection
Physical transfection involves stimulating the surface of the cell to introduce the nucleic acid through the cell membrane. Methods include electroporation, microinjection, and laser processing, with electroporation being the most common. During electroporation, the nucleic acids and the cells are suspended in a conductive solution and then clamped between an anode and a cathode. Electric pulses are then applied continuously to create small holes in the cell membrane. These holes are then used to transfer extracellular nucleic acids with an electrical charge—such as DNA and RNA—through the cell membrane.
物理转染具有较高的转染效率,并且比化学和生物转染方法更容易。但是,电脉冲可能会导致细胞死亡,电脉冲制成的孔可能不会接近,导致细胞毒性,并且需要昂贵的专用设备。
提高转染效率
Transfection efficiency is the efficiency of nucleic acid uptake by the target cells. This section introduces various factors to consider for improving the efficiency of transfection.
- 细胞活力和传播
- 转染前的细胞活力应至少为90%。转染后的24小时内也可以进行传播(细胞从培养物转移到新培养基)的地方。另外,请注意,过度传言可能会对转染效率产生不利影响,因此应仔细注意通道号。
- 细胞cultures and reagents
-
The confluency, which indicates the ideal proliferation status of a cell for transfection, varies depending on the testing purpose, method, cell type, etc. If the confluence is too high, nucleic acid uptake may be insufficient, or transgene expression may be reduced. If the confluence is too low, intercellular contact may not occur, preventing proliferation.
转染生长细胞的最佳培养基取决于细胞,试剂和细胞型血清。当将DNA引入干细胞中时,例如,能够将核酸引入胚胎干细胞(ES细胞),诱导多能干细胞(IPS细胞),人类成人干细胞和其他高效效率和低毒性细胞的试剂,诱导的多能干细胞(IPS细胞)和其他类型的细胞被使用。当将RNA引入干细胞中时,使用了能够引入siRNA(小干扰RNA),mRNA(Messenger RNA)和DSRNA(双链RNA)的试剂,同时使用保护干细胞免受降解。 - Transfection method selection
-
转染方法包括化学,生物学和物理转染。化学和物理转染通常用于靶向培养细胞的靶向转染。生物转染可有效转染培养的细胞和动物细胞。但是,化学转染可能会导致转染效率或化学毒性的变化,具体取决于细胞类型和条件,并且某些细胞可能很难选择性转染。同时,物理转染需要特殊的仪器和设备,核酸很容易受损。生物学转染还具有污染的风险,插入突变时,将治疗基因引入细胞染色体以及基于免疫力的失活。
Because each method has disadvantages, selecting a transfection method with a high transfection efficiency and high reproducibility for the specific combination of transfection cells and target cells is essential. - 其他因素
-
Transfection efficiency also varies depending on whether serums or antibiotics are used. For example, when introducing DNA, culturing the cells in a medium with serum increases efficiency. Performing transient transfection in a medium with antibiotics also increases transfection efficiency.
但是,抗生素还会引起细胞毒性,从而对细胞产生不利影响并降低转染效率。此外,DNA和RNA矢量都可以用于瞬时转染,但只能将DNA矢量用于稳定转染。
Measuring transfection efficiency
测量转染效率的一种一般方法是使用荧光显微镜。转染效率是通过计算观察到的细胞总数和表达荧光的细胞数量并评分这些值来衡量的。
转染效率测量
在基因引入期间,将用生长培养基制备的细胞悬浮液播种,以适当的细胞密度进入井板并孵化。然后,将制备的复合物添加到细胞中,摇动井板,然后在孵化器中再次孵育24小时。当孵育结束时,观察细胞的表达以测量转染效率。通常使用荧光染料和荧光显微镜测量转染效率。
转染效率测量挑战
Conventional fluorescence microscopes pose various problems and are often difficult to use.
首先,观察荧光需要将样品带到暗室并在那里执行实验,从而导致可操作性极差。精确提取细胞轮廓也很困难,阻碍了细胞数量的准确计数。
此外,为了计数和分析单元格的数量,需要与荧光显微镜系统分开的软件,从而导致许多客户注意执行平滑分析的困难。
The BZ-X800 All-in-One Fluorescence Microscope includes a built-in specimen enclosure that allows users to perform fluorescence imaging in a brightly lit room. The BZ-X800 accurately extracts and quantifies phase-contrast image contours, even for cultured cells with subtle luminance differences, and allows users to specify a cell mask area for measuring the number of measurement targets and the surface area ratio. The BZ-X800 can also measure cell expression levels for quantitative measurement of transfection efficiency without needing separate software.
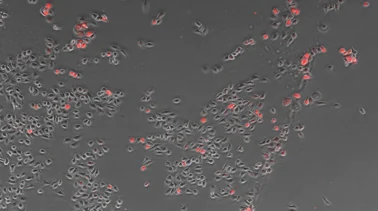
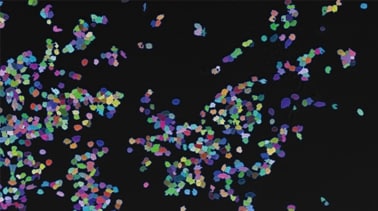
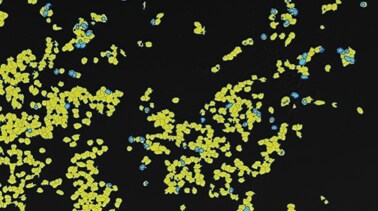
- 细胞
- 903
- 表达
- 89
- 效率
- 9.9%
Objective lens: CFI Plan Fluor DL 10x
Using the All-in-One Fluorescence Microscope BZ-X800
- 因为不要求暗室ed, easy fluorescence imaging can be done in any laboratory setting. A low-photobleach imaging mode can be used to minimize damage to the cells.
- 相比对比度和荧光图像都可以捕获,然后覆盖以获得更全面的视图。
- 无论是使用相比还是荧光图像,我们的专有测量软件都可以快速提取细胞的轮廓并准确计数或测量其特性。
- 杂化细胞计数可用于提取细胞中包含的荧光蛋白,并通过使用细胞作为掩模区域进行计数。
- Here are some examples of using the All-in-One Fluorescence Microscope BZ-X800 in front-line research.
- [Myelodysplastic Syndromes (MDS)] Stitching, Sectioning and the Z-Stack Function as Decisive Arguments for the Acquisition of the BZ Fluorescence Microscope at the University Hospital of Düsseldorf
- [Neuropathology] The perfect solution for everyday patient diagnostics and clinical research at the Institute of Neuropathology in the Charité hospital in Berlin
- [再生医学] BZ系列为神经干细胞和脊柱观察提供了必需的成像
- [基因疗法]改善基因治疗药物的开发研究
- [Heart Disease Treatment] Developing Cell Sheets for Myocardial Regenerative Treatments
- [癌症治疗]自动荧光显微镜改变了诱导癌症干细胞研究的过程
- [免疫系统] BZ系列有助于理解哮喘的病理模型
- [生物材料]通过紧凑,用户友好的显微镜提高研究效率